In the last 10 years the genetic engineering of bacteria as well as mammalian cell lines allow to design and produce extracellular vesicles decorated with specific protein antigens that can be used as vaccine Outer membrane vesicles (OMVs) are released spontaneously during growth by many Gram-negative bacteria. candidates, as immnunogen for the production of monoclonal antibodies for those antigens (eg intregral membrane proteins) that are difficult to produce in soluble recombinant forms.
Extracellular vesicles are released spontaneously during growth of Gram-negative bacteria as well as mammalian cell lines (eg. HEK293).
For some applications wild-type strains can be used directly to produce an extracellular vesicle but, in most cases, genetic engineering of the expression Host is required not only to induce the over-expression of specific single on multiple antigens but also to improve vesicles productivity and safety.
In gram-negative bacteria several genetic modifications able to improve vesicles production (eg. TolR, OmpA deleted strain) as well as modifying the synthesis of a LPS carrying (e.g msbB, pagP and other mutants) and reduce vesicle reactogenicity were already identified and tested.
Therefore, even if the mammalian extracellular vesicles (e.g exosomes) have been more extensively studied than bacterial extracellular vesicles, one of the challenges the limit the use of mammalian vesicles as scaffold for antigen expression is their low production yield.
Therefore, similarly to what happened 20 years ago at the beginning of recombinant protein expression age, the Bacteria thanks to their rapid proliferative abilities, process scalability of their culture methods and gene editing ability has been more extensively applied for extracellular vesicle production.
However, in the last 20 years several new technological improvements as:
1) The adaptation of several cell lines to suspension cultures;
2) The development of more efficient transfection agents and new gene editing technologies:
3) The development of serum free media where the cells are able to growth at high cell density;
Improve drastically the performances of the mammalian expression systems and make it not only very interesting as platform for production of recombinant soluble proteins but also as factory of engineered extracellular vesicles.
Chinese hamster ovary (CHO) cells are widely used host cells for recombinant protein production and currently the most commonly utilized mammalian organism in large scale bio-pharmaceutical production and some recent papers (1,2) report their ability to produce extracellular vesicles.
As I already show you in the ProteoCool Pill#8 the ExpiCHO cell line is a CHO derivate that thanks to its ability to growth very high cell density (8-10 milion cells/ml) and high transfection efficiency result in high yields of recombinant antibody and protein production.
In this post I would like to briefly share with you some preliminary result that suggest as ExpiCHO may become also a promising platform for production of engineered extracellular vesicles. I would like to point out as the fact that ExpiCHO growth very well in serum free media is essential for production of engineered extracellular vesicles since the serum contain a large amout of unwanted empty vesiscles that may contaminate our preparation and vesicle serum depletion is time consuming and difficult to scale up.
This trials were performed by comparing the extracellular production ability of commercial ExpiCHO empty cells with an antigen over expressing Expo-CHO cell line derivate that was generated (data not shown) using the Flp-In cloning system.
Since Expi-CHO Flp-In cell line are not commercially available, first of all, an ExpiCHO Flp-In cell line was generated by transfection of ExpiCHO with pFRT/lacZeo vector and the positive clone were isolated after growth under Zeocin selection.
Afterwards the GPCR expressing ExpiCHO cell line was generated by co-tranfection of GPCR-pcdna5/FRT and pOG44 vectors and the positive clone were isolated after growth under Hygromicyn selection.
1° Vesicles production trial:
Comparison of 2 different growing protocols for the the empty ExpiCHO cell line.
Protocol Overview
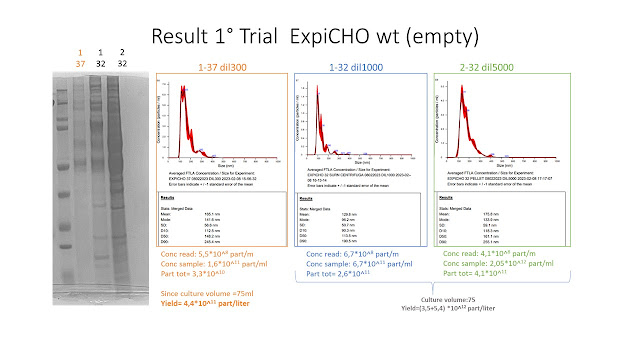
After re-suspension with PBS the Vesicles were subjected to SDS-page and Nanotracking particle analisys (Nanosight- Malvern)
Even if the NTA results do reveal important differences between the vesicles produced at 37°C and 32°C, the presence of the strong yellow colour (which suggest the presence of some contaminant) as well us the partial resuspension at 32°C lead us to prefer the condition n°1.
2° Vesicles production trial:
Vesicle production with condition 1 using empty and GPCR expression ExpiCHO
Even it those results are really preliminary, the fact that extracellular vesicle expressing a GPCR could be successfully isolated in good amount (about 10^10 vesicles/liter) suggest that ExpiCHO may represent a promising platform for production of engineered extracellular vesicle suitable as vaccine component or immunogen for the production of new monoclonal antibodies targeting specific transmembrane antigens.