Static light scattering (SLS) is a technique to measure absolute molecular weight using the relationship between the intensity of light scattered by a molecule and its molecular weight and size.
Some SLS technologies exist: multiangle light scattering (MALS), right-angle light scattering (RALS), low-angle light scattering (LALS) and RALS/LALS hybrid systems
MALS or RALS/LALS when coupled with other detectors (eg UV-vis, RI, densitometry, fluorimeter) in an advanced GPC-SEC system can be applied to investigate solution properties, stability testing and process development for different kind of conjugated and unconjugateed biologics
Light scattering detectors (low-angle LALS, right-angle RALS, or multi-angle MALS) are very sensitive to the presence of particulates, when those are used for molecular weight detection in chromatography. Unfortuantelly even the best columns can shed some particulates from their packing material. Although undetected by most conventional detectors, such as UV and RI, these particles scatter significant amounts of light, produce noise that affect the light scattering signals and baselines. To mitigate this, the MALS, LALS/RALS sistems include an in-line coloumn filter that can significantly improve the quality and thus the accuracy of both the data and results.
In this post i would like to share with you some data acquired loading different mabs in a Cytiva Supedex200 increase 10/30 coloumn in the OMNISEC instrument equiped wih the LALS/RALS detector in the presence/absence of post coloumn filter.
First of all we compared the baseline signals that can be obtained
- Without filter;
- With a 0,2um nylon filter;
- With 0,2um cellulose filter;
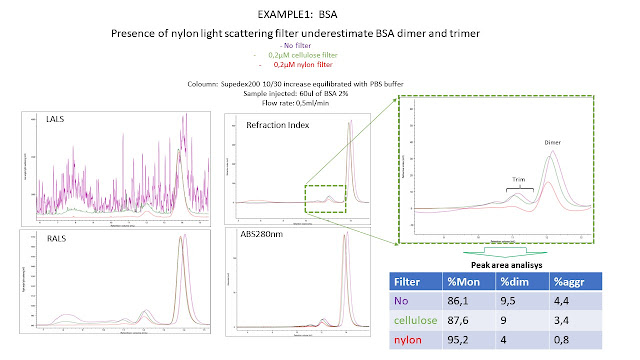
The baseline signals suggest that nylon filter is the best in terms of signal/noise ratio
Is it really the best choiche for analisys of protein/antibody preparations?
To assess it, a 2% BSA standard and 2 different purified monoclonal antibodies (igG1 human) were loaded in the superdex200 10/30 increase coloumn and analized with the OMNISEC instrumentation
In all the 3 cases, the Nylon filter guarantee the best signal/noise ratio at LALS but it seems to mask the presence of a significant fraction of high molecular weight proteins.
This was most evident for the mabs, since the dimeric-trimeric and MW aggregates were not detected, even if at UV and RI, using the nylon filter.
ThIs differences may lead to overtimating the quality of a certain mab or protein preparation and it can lead to false positive results in case of stability studies that would like investigate the mab/protein aggregation propensity under stress (as acid pH, 37°C, freeze/thaw)
For this kind of studies cellulose filter seem to be the best compromise between quality of LALS signal and recovery of the protein polimers.
As a general comment: Often the diffence is in the details! It is important be able to critically review the Positive results to distinguish the real positives than false positives!
Suggested links:
https://www.materials-talks.com/how-to-change-the-light-scattering-post-column-filter-membrane/